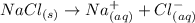When an ionic compound dissolves in water:
a. the negative ends of water molecules surround...

When an ionic compound dissolves in water:
a. the negative ends of water molecules surround the positive ions.
b. the negative ends of water molecules surround both the negative and the positive ions.
c. the positive ends of water molecules surround the positive ions.
d. the negative ends of water molecules surround the negative ions.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1

Chemistry, 23.06.2019 11:00
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2
You know the right answer?
Questions




Chemistry, 05.09.2020 16:01

Mathematics, 05.09.2020 16:01


English, 05.09.2020 16:01

Mathematics, 05.09.2020 16:01

Mathematics, 05.09.2020 16:01








Spanish, 05.09.2020 16:01



Social Studies, 05.09.2020 16:01