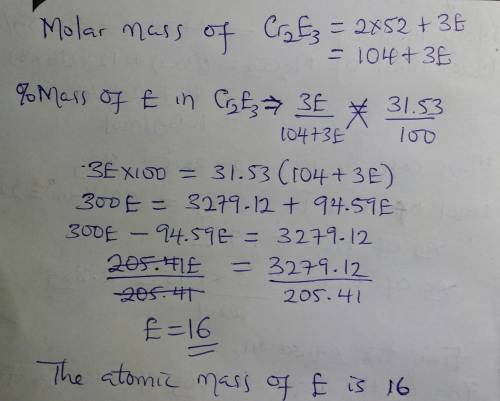Chemistry, 28.01.2020 19:41 izzy201995
Abinary compound created by reaction of chromium and an unknown element e contains 68.47% cr and 31.53% e by mass. if the formula of the compound is cr2e3, calculate the atomic mass of e.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 23.06.2019 06:00
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1


You know the right answer?
Abinary compound created by reaction of chromium and an unknown element e contains 68.47% cr and 31....
Questions




Mathematics, 12.11.2020 21:50

Biology, 12.11.2020 21:50

Mathematics, 12.11.2020 21:50

History, 12.11.2020 21:50



History, 12.11.2020 21:50

English, 12.11.2020 21:50

Mathematics, 12.11.2020 21:50

Mathematics, 12.11.2020 21:50


SAT, 12.11.2020 21:50


Mathematics, 12.11.2020 21:50

German, 12.11.2020 21:50


Mathematics, 12.11.2020 21:50