Chemistry, 28.01.2020 20:51 vjackie101ov3kju
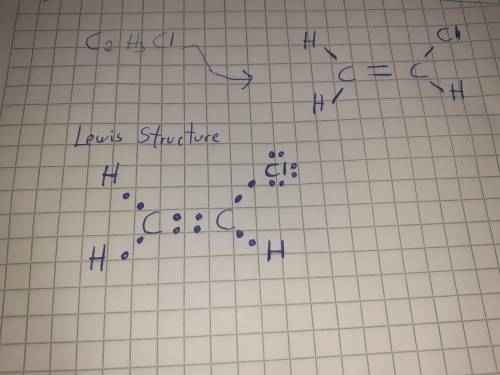
Draw a lewis structure for c2h3cl. show all unshared electron pairs. none of the atoms bears a formal charge, and all atoms have octets (except for hydrogen atoms, which have duets).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3


Chemistry, 23.06.2019 09:30
The mass of a proton is approximately equal to the mass of
Answers: 1

Chemistry, 23.06.2019 11:30
The dashed segment of the plotted experiment in the graph in the l
Answers: 3
You know the right answer?
Draw a lewis structure for c2h3cl. show all unshared electron pairs. none of the atoms bears a forma...
Questions



Business, 18.12.2020 17:40

Mathematics, 18.12.2020 17:40





Geography, 18.12.2020 17:40

Mathematics, 18.12.2020 17:40



History, 18.12.2020 17:40



Mathematics, 18.12.2020 17:40

English, 18.12.2020 17:40


Computers and Technology, 18.12.2020 17:40

World Languages, 18.12.2020 17:40