Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 23.06.2019 01:00
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1

Chemistry, 23.06.2019 07:00
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table?
Answers: 2
You know the right answer?
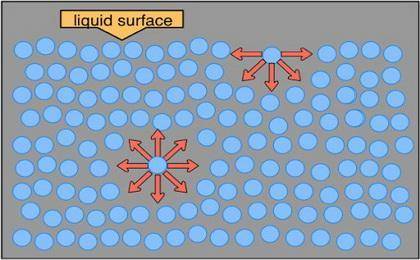
The surface tension of water is 7.28 ✕ 10−2 j/m2 at 20°c. predict whether the surface tension of hep...
Questions

Biology, 03.04.2020 01:58


Chemistry, 03.04.2020 01:58

Mathematics, 03.04.2020 01:58


History, 03.04.2020 01:58



Mathematics, 03.04.2020 01:59


Mathematics, 03.04.2020 01:59






Medicine, 03.04.2020 01:59

Mathematics, 03.04.2020 01:59

English, 03.04.2020 01:59