Chemistry, 29.01.2020 02:51 GreenHerbz206
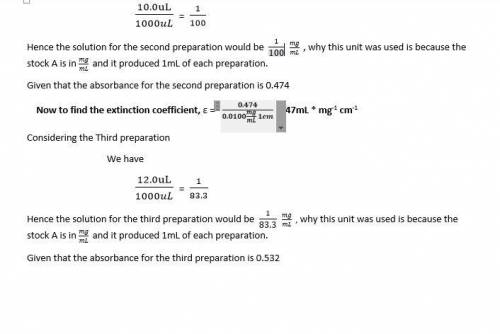
Calculate the extinction coefficient where the concentration is in mg/ml and the path length is 1 cm. what dilutions of the stock are each of the prepared solutions (i. e., 1/x)?
the molecular weight of a is 290 g/mole.
re-calculate the extinction coefficient with the concentration in mm. note that the newly calculated extinction coefficient will contain an mm-1 term.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
Calculate the extinction coefficient where the concentration is in mg/ml and the path length is 1 cm...
Questions








History, 22.07.2019 09:00


History, 22.07.2019 09:00


History, 22.07.2019 09:00



History, 22.07.2019 09:00

History, 22.07.2019 09:00



Social Studies, 22.07.2019 09:00

Social Studies, 22.07.2019 09:00