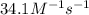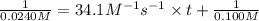Chemistry, 29.01.2020 03:52 njohns5327home
At a certain temperature the rate of this reaction is second order in nh4oh with a rate constant of 34.1 m^-1*s^-1:
nh4oh(aq) > nh3(aq) + h2o (aq)
suppose a vessel contains nh4oh at a concentration of 0.100m. calculate how long it takes for the concentration of nh4oh to decrease to 0.0240 m. you may assume no other reaction is important. round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1


Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1
You know the right answer?
At a certain temperature the rate of this reaction is second order in nh4oh with a rate constant of...
Questions






English, 06.06.2020 14:57





English, 06.06.2020 14:57


Mathematics, 06.06.2020 14:57

Computers and Technology, 06.06.2020 14:57

Chemistry, 06.06.2020 14:57





History, 06.06.2020 14:57

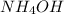 to decrease to 0.0240 M.
to decrease to 0.0240 M.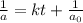
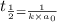
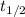 = half life of reaction
= half life of reaction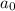 = initial concentration
= initial concentration 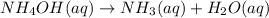
![[NH_4OH]=a_o=0.100 M](/tpl/images/0480/1114/fa6e4.png)
![[NH_4OH]=a=0.0240 M](/tpl/images/0480/1114/c0e10.png)