
Chemistry, 29.01.2020 04:42 choiboiqg5755
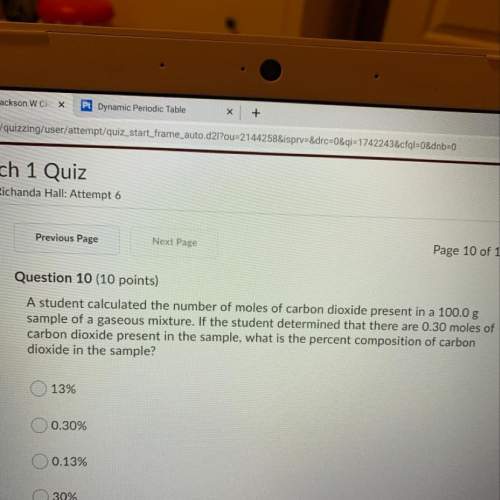
Astudent calculated the number of moles of carbon dioxide present in a 100.0g sample of a gaseous mixture. if the student determined that there are 0.30 moles of carbon dioxide present in the sample, what is the percent composition of carbon dioxide in the sample?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1
You know the right answer?
Astudent calculated the number of moles of carbon dioxide present in a 100.0g sample of a gaseous mi...
Questions

Mathematics, 15.06.2021 17:50

Mathematics, 15.06.2021 17:50

Mathematics, 15.06.2021 17:50



Biology, 15.06.2021 17:50






Mathematics, 15.06.2021 17:50



Mathematics, 15.06.2021 17:50


Mathematics, 15.06.2021 17:50

Mathematics, 15.06.2021 17:50

Biology, 15.06.2021 17:50

Mathematics, 15.06.2021 17:50



