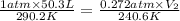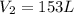Chemistry, 29.01.2020 05:41 allieliquori
Aballoon filled with 50.3 l of he at 17.2°c and 1.00 atm rises to a height in the atmosphere where the pressure is 207 torr and the temperature is − 32.4°c.
part a) what is the final volume of the balloon? assume that the pressure inside and outside the balloon have the same value express your answer with the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The ph of carrots are 5.0 how it is classified a.acidic b.basic c.indicator d.neutral
Answers: 2

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
Aballoon filled with 50.3 l of he at 17.2°c and 1.00 atm rises to a height in the atmosphere where t...
Questions

Mathematics, 08.02.2021 20:10



World Languages, 08.02.2021 20:10

Mathematics, 08.02.2021 20:10


Physics, 08.02.2021 20:10


Social Studies, 08.02.2021 20:10

English, 08.02.2021 20:10

Social Studies, 08.02.2021 20:10

Mathematics, 08.02.2021 20:10



History, 08.02.2021 20:10


Physics, 08.02.2021 20:10

Mathematics, 08.02.2021 20:10

Mathematics, 08.02.2021 20:10

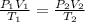
 = initial pressure of gas = 1.00 atm
= initial pressure of gas = 1.00 atm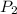 = final pressure of gas = 207 torr = 0.272 atm (760torr=1atm)
= final pressure of gas = 207 torr = 0.272 atm (760torr=1atm)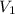 = initial volume of gas = 50.3 L
= initial volume of gas = 50.3 L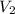 = final volume of gas = ?
= final volume of gas = ?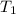 = initial temperature of gas =
= initial temperature of gas = 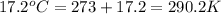
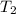 = final temperature of gas =
= final temperature of gas =