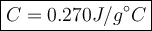2. a 237 g piece of molybdenum, initially at 100.0 °c, was dropped into 244 g of
water at 10.0...

Chemistry, 29.01.2020 14:40 alonnachambon
2. a 237 g piece of molybdenum, initially at 100.0 °c, was dropped into 244 g of
water at 10.0°c. when the system came to thermal equilibrium, the temperature
was 15.3°c. what is the specific heat capacity of molybdenum? the specific heat
capacity of water is 4.184 j/g. k. (2.5 points)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1
You know the right answer?
Questions


Mathematics, 23.02.2021 01:00

Geography, 23.02.2021 01:00


History, 23.02.2021 01:00

English, 23.02.2021 01:00


Mathematics, 23.02.2021 01:00


Mathematics, 23.02.2021 01:00

Mathematics, 23.02.2021 01:00


Mathematics, 23.02.2021 01:00



History, 23.02.2021 01:00

Mathematics, 23.02.2021 01:00



Mathematics, 23.02.2021 01:00