how many moles of hydrogen are needed to produce 13.78 mol of ethane?

Chemistry, 22.09.2019 13:30 versaceblooper
C2h2+2h2 yields c2h6
how many moles of hydrogen are needed to produce 13.78 mol of ethane?
pls

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉more accurate estimates can be made with the van der waals equationí‘ťí‘ť=푛푛푛푛푛푛푉푉â’푛푛푟푟â’푞푞푛푛2푉푉2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1


Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
C2h2+2h2 yields c2h6
how many moles of hydrogen are needed to produce 13.78 mol of ethane?
how many moles of hydrogen are needed to produce 13.78 mol of ethane?
Questions

Mathematics, 26.01.2021 17:10


Mathematics, 26.01.2021 17:10




Mathematics, 26.01.2021 17:10

Geography, 26.01.2021 17:10


Mathematics, 26.01.2021 17:10


Mathematics, 26.01.2021 17:10

Mathematics, 26.01.2021 17:10



Chemistry, 26.01.2021 17:10




History, 26.01.2021 17:10

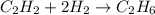
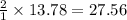 moles of hydrogen
moles of hydrogen 

