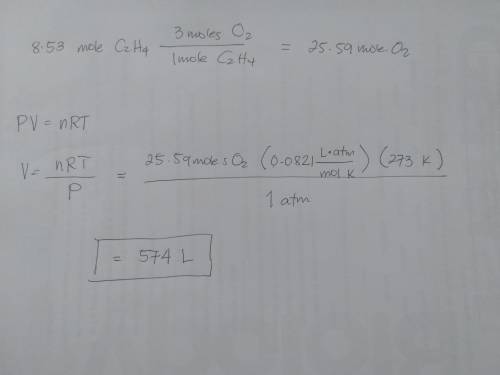
Chemistry, 02.02.2020 12:42 DonovanBaily42
What volume of oxygen at stp is needed to fully react with 8.53 mol of c2h4?
c2h4 reacts with o2, according to the following equation:
c2h4(g) + 3o2(g) → 2co2(g) + 2h2o(g)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 23.06.2019 08:00
Which term means two or more atoms that share electrons in a chemical bond? a. hydrogen bond b. moleculec. ionic bondd. element amd you
Answers: 3
You know the right answer?
What volume of oxygen at stp is needed to fully react with 8.53 mol of c2h4?
c2h4 reacts with...
c2h4 reacts with...
Questions







Mathematics, 14.08.2021 21:30



Mathematics, 14.08.2021 21:30

Mathematics, 14.08.2021 21:30


Physics, 14.08.2021 21:30

History, 14.08.2021 21:30

History, 14.08.2021 21:30

Business, 14.08.2021 21:30

Biology, 14.08.2021 21:30


Mathematics, 14.08.2021 21:30

Mathematics, 14.08.2021 21:30