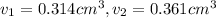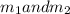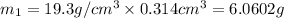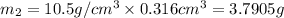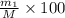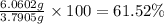Chemistry, 11.11.2019 06:31 kimloveswim
Gold is alloyed(mixed) with other metals to increase its hardness in making jewelry. (a) consider a piece of gold jewelry that weighs 9.85 g and has a volume of 0.675 cm3. the jewelry contains only gold and silver, which have densities of 19.3 g/cm3 and 10.5 g/cm3, repectively. if the total volume of the jewelry is the sum of the volumes of the gold and silver that it contains. calculate the percentage of gold(by mass) in the jewelry. (b) the relative amount of gold in an alloy is commonly expressed in units of karats.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2


Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1
You know the right answer?
Gold is alloyed(mixed) with other metals to increase its hardness in making jewelry. (a) consider a...
Questions

Computers and Technology, 19.04.2022 04:10



Chemistry, 19.04.2022 04:50




Biology, 19.04.2022 05:20




Mathematics, 19.04.2022 05:20


Mathematics, 19.04.2022 05:20

Mathematics, 19.04.2022 05:20





Mathematics, 19.04.2022 06:40

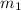
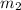
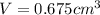
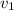
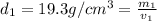
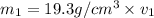
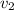
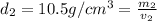
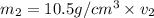
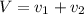
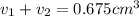 ..(1)
..(1)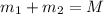
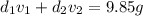
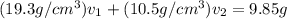 ..(2)
..(2)