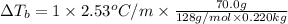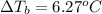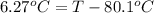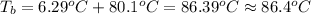Calculate the boiling point of a solution prepared by dissolving 70.0 g of naphthalene, c10h8 (a nonvolatile nonelectrolyte), in 220.0 g of benzene, c6h6. the kb for benzene = 2.53oc/m. the boiling point of pure benzene is 80.1oc.. ans: 86.4 degrees celsius. i did this so far. 1)70g c10h8(1mol c10h8/128gc10h8)= .546mol c10h8. benzene)=2.482m. 3) (2.482)(2.53 c/m)=6.289. i'm not sure if i am starting this off right, can anyone me get the correct answer? ans ty!

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
This graph gives information on changes in sea ice extent in the arctic ocean over a 30-year span. the overall trend shows in the ice extent. to address the trend, scientists need to ask themselves, one direct consequence of the trend is that
Answers: 1

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1


Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1
You know the right answer?
Calculate the boiling point of a solution prepared by dissolving 70.0 g of naphthalene, c10h8 (a non...
Questions

Mathematics, 06.05.2021 16:50




Mathematics, 06.05.2021 16:50


Arts, 06.05.2021 16:50


Mathematics, 06.05.2021 17:00

History, 06.05.2021 17:00

Mathematics, 06.05.2021 17:00



Mathematics, 06.05.2021 17:00





Mathematics, 06.05.2021 17:00

Mathematics, 06.05.2021 17:00

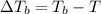
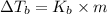
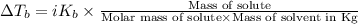
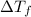 =Elevation in boiling point
=Elevation in boiling point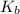 = Boiling point constant of solvent = 2.53 °C/m(benzene)
= Boiling point constant of solvent = 2.53 °C/m(benzene)