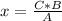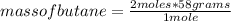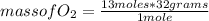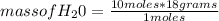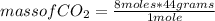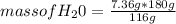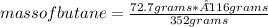The mass of water produced when 7.36g of butane reacts with excess oxygen is 11.42 grams.
The mass of butane needed to produce 72.7g of carbon dioxide is 23.96 grams.
Explanation:
The rule of three or is a way of solving problems of proportionality between three known values and an unknown value, establishing a relationship of proportionality between all of them. That is, what is intended with it is to find the fourth term of a proportion knowing the other three. Remember that proportionality is a constant relationship or ratio between different magnitudes.
If the relationship between the magnitudes is direct, that is, when one magnitude increases, so does the other, the direct rule of three must be applied. To solve a direct rule of three, the following formula must be followed:
A ⇒ B
C ⇒ x
So,
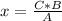
In this case the rule of three will be used for the calculations. You have the reacction
2 C₄H₁₀(g) + 13 O₂(g) ⇒ 10 H₂O(g) + 8 CO₂(g)
Calculate the mass of water produced when 7.36g of butane reacts with excess oxygen
First of all you must know the mass that reacts and that is formed of each compound. For this, we must know the atomic mass of each element that forms the compound:
C: 12 g/molH: 1 g/molO: 16 g/mol
Taking into account the present quantity of each element in the compounds, it is possible to calculate its mass:
C₄H₁₀: 4*12 g/mol +10*1 g/mol= 58 g/molO₂: 2*16 g/mol= 32 g/molH₂O: 2*1 g/mol +16 g/mol= 18 g/molCO₂: 12 g/mol +2*16 g/mol= 44 g/mol
Using the stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction) and the mass of each compound, you can use a simple rule of three to determine the mass that reacts by stoichiometry of each compound. The rule of three is used as follows for the calculation of the mass of butane C₄H₁₀: if one mole of butane contains 58 g, how much mass does it contain 2 moles of butane?
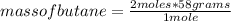
mass of butane=116 g
Following an approach similar to that proposed for butane, it is possible to calculate the stoichiometrically reacting mass of O₂ oxygen, and the stoichiometrically produced mass of H₂O (water) and CO₂ (carbon dioxide). It is then obtained:
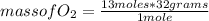
mass of O₂= 416 g
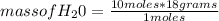
mass of H₂O= 180 g
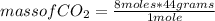
mass of CO₂= 352 g
Then, knowing the mass that reacts and is produced from each compound by stoichiometry, it is possible to calculate the mass of water produced when 7.36 g of butane react with excess oxygen. In this case the amount of oxygen reacting mass is not important, since being in excess the compound that is consumed first is butane. For the calculation of the mass of water produced, a rule of three is used: if 116 g of butane produce 180 g of water, how many grams of water will produce 7.36 g of butane?
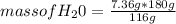
mass of H₂O= 11.42 grams
So, the mass of water produced when 7.36g of butane reacts with excess oxygen is 11.42 grams.
Calculate the mass of butane needed to produce 72.7g of carbon dioxide
As in the previous case, knowing the stoichiometric mass of the reaction, it is possible to calculate the mass of butane that must react using a rule of three: if 116 g of butane produces 352 g of carbon dioxide, how many grams of butane are needed to produce 72.7 grams of carbon dioxide?
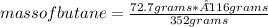
mass of butane= 23.96 grams
So, the mass of butane needed to produce 72.7g of carbon dioxide is 23.96 grams.