Chemistry, 04.02.2020 05:51 elijahjacksonrp6z2o7
How much energy must be removed from a 125 g sample of benzene (molar mass= 78.11 g/mol) at 425.0 k to liquify the sample and lower the temperature to 335.0 k? the following physical data may be useful.
hvap = 33.9 kj/molhfus = 9.8 kj/mol
cliq = 1.73 j/g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1

Chemistry, 23.06.2019 08:00
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1
You know the right answer?
How much energy must be removed from a 125 g sample of benzene (molar mass= 78.11 g/mol) at 425.0 k...
Questions



Biology, 19.09.2019 06:30



Chemistry, 19.09.2019 06:30




Mathematics, 19.09.2019 06:30




History, 19.09.2019 06:30

Mathematics, 19.09.2019 06:30

Chemistry, 19.09.2019 06:30



Health, 19.09.2019 06:30

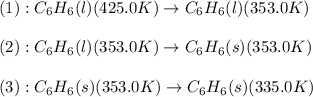
![\Delta H=[m\times c_{p,l}\times (T_{final}-T_{initial})]+m\times \Delta H_{fusion}+[m\times c_{p,s}\times (T_{final}-T_{initial})]](/tpl/images/0499/2996/53889.png)
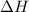 = heat available for the reaction =
= heat available for the reaction = 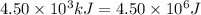
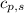 = specific heat of solid benzene =
= specific heat of solid benzene = 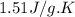
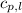 = specific heat of liquid benzene =
= specific heat of liquid benzene = 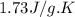
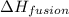 = enthalpy change for fusion =
= enthalpy change for fusion = 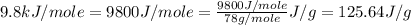
![\Delta H=[125g\times 1.73J/g.K\times (353-425)K]+125g\times -125.64J/g+[125g\times 1.51J/g.K\times (335-353)K]](/tpl/images/0499/2996/d60b8.png)