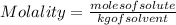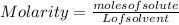The equation used to calculate the change in freezing point (δtf) of a substance is:
δtf = k...

The equation used to calculate the change in freezing point (δtf) of a substance is:
δtf = kfm
where kf is the freezing point depression constant and m is the molality of the solution. which of the statements explains why molality is used instead of molarity in this equation?
a. molality does not appear in many equations, so it is used here to distinguish this equation from other similar ones.
b. as the temperature of a solution changes, its volume will also change, which will affect its molarity but not its molality.
c. in solutions, moles are not directly related to grams and the freezing point of a solution is dependent solely on the number of grams of solute.
d. the equation was originally published with m as a typo, rather than m, but the values are close enough that the equation is still valid.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

You know the right answer?
Questions






Mathematics, 17.10.2019 21:30

Mathematics, 17.10.2019 21:30

Health, 17.10.2019 21:30

Biology, 17.10.2019 21:30

English, 17.10.2019 21:30



History, 17.10.2019 21:30

Health, 17.10.2019 21:30

Biology, 17.10.2019 21:30




Social Studies, 17.10.2019 21:30

Mathematics, 17.10.2019 21:30