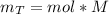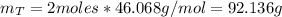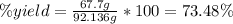Chemistry, 05.02.2020 21:41 NNopeNNopeNNope
Consider the fermentation reaction of glucose: c6h12o6 → 2c2h5oh + 2co2 a 1.00-mol sample of c6h12o6 was placed in a vat with 100 g of yeast. if 67.7 g of c2h5oh was obtained, what was the percent yield of c2h5oh?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1

Chemistry, 23.06.2019 09:50
T(s) in2os] (m) 0 185 2.39 546 1.90 725 1.70 the decomposition of n205 can be described by the equation 2.68 given these data for the reaction at 45°c in carbon tetrachloride solution, calculate the average rate of reaction for each successive time interval. ntr s to 185 s 185 s to 546 s 546 s to 725 s number number number reaction rate: m/s m/s m/s
Answers: 1
You know the right answer?
Consider the fermentation reaction of glucose: c6h12o6 → 2c2h5oh + 2co2 a 1.00-mol sample of c6h12o...
Questions

Physics, 16.10.2019 03:00

Mathematics, 16.10.2019 03:00






Mathematics, 16.10.2019 03:00



Biology, 16.10.2019 03:00



Mathematics, 16.10.2019 03:00

Biology, 16.10.2019 03:00


Biology, 16.10.2019 03:00


Mathematics, 16.10.2019 03:00

Mathematics, 16.10.2019 03:00

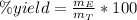
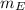 : is the obtained mass of C₂H₅OH = 67.7g and
: is the obtained mass of C₂H₅OH = 67.7g and 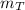 : is the theoretical mass of C₂H₅OH.
: is the theoretical mass of C₂H₅OH.