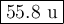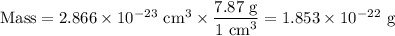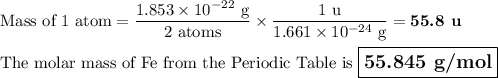Metallic iron has a body-centered cubic lattice with all atoms at lattice points and a unit cell whose edge length is 286.6 pm. the density of iron is 7.87 g cm–3 . what is the mass of an iron atom? compare this value with the value you obtain from the molar mass

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
Metallic iron has a body-centered cubic lattice with all atoms at lattice points and a unit cell who...
Questions

Mathematics, 08.06.2021 17:00

English, 08.06.2021 17:00


Mathematics, 08.06.2021 17:00

Mathematics, 08.06.2021 17:00


Computers and Technology, 08.06.2021 17:00


Mathematics, 08.06.2021 17:00

English, 08.06.2021 17:00

Mathematics, 08.06.2021 17:00

Arts, 08.06.2021 17:00


English, 08.06.2021 17:00


Biology, 08.06.2021 17:00

Mathematics, 08.06.2021 17:00