
Chemistry, 06.02.2020 02:44 ddddre4460
An unknown compound contains only c , h , and o . combustion of 9.30 g of this compound produced 22.7 g co 2 and 9.29 g h 2 o . what is the empirical formula of the unknown compound? insert subscripts as needed. empirical formula: ?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
An unknown compound contains only c , h , and o . combustion of 9.30 g of this compound produced 22....
Questions


Law, 12.05.2021 15:30


Geography, 12.05.2021 15:30




History, 12.05.2021 15:30

Mathematics, 12.05.2021 15:30




Mathematics, 12.05.2021 15:30

Physics, 12.05.2021 15:30





Arts, 12.05.2021 15:30

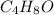
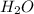 = 9.29 g /18 g/mol = 0.51611 moles
= 9.29 g /18 g/mol = 0.51611 moles
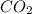 = 22.7 g /44.01 g/mol = 0.5158 moles
= 22.7 g /44.01 g/mol = 0.5158 moles


