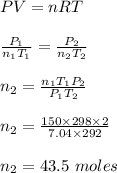Chemistry, 10.02.2020 05:39 Sonicawesomeness
A steel cylinder contains 150.0 mol argon gas at a temperature of 25°C and a pressure of 7.04 MPa. After some argon has been used, the pressure is 2.00 MPa at a temperature of 19°C. What mass (g) of argon remains in the cylinder?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3
You know the right answer?
A steel cylinder contains 150.0 mol argon gas at a temperature of 25°C and a pressure of 7.04 MPa. A...
Questions



Mathematics, 08.12.2020 18:00

Mathematics, 08.12.2020 18:00




Mathematics, 08.12.2020 18:00


Biology, 08.12.2020 18:00



Engineering, 08.12.2020 18:00

Mathematics, 08.12.2020 18:00

English, 08.12.2020 18:00

Mathematics, 08.12.2020 18:00

Mathematics, 08.12.2020 18:00

Social Studies, 08.12.2020 18:00