
Chemistry, 10.02.2020 22:28 rosie20052019
A solution is prepared by adding 91.3 g of sodium bromide [NaBr], to 115. g of water. Determine whether the solution is saturated, unsaturated, or if a precipitate will form given that the solubility of NaBr is 9.19 m.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2
You know the right answer?
A solution is prepared by adding 91.3 g of sodium bromide [NaBr], to 115. g of water. Determine whet...
Questions

Mathematics, 09.03.2021 04:30





Mathematics, 09.03.2021 04:30



English, 09.03.2021 04:30

English, 09.03.2021 04:30




Chemistry, 09.03.2021 04:30

History, 09.03.2021 04:30

Mathematics, 09.03.2021 04:30

Spanish, 09.03.2021 04:30


Business, 09.03.2021 04:30

Physics, 09.03.2021 04:30

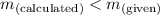 ; the solution is unsaturatedWhen
; the solution is unsaturatedWhen 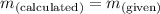 ; the solution is saturatedWhen
; the solution is saturatedWhen 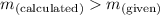 ; precipitate will form
; precipitate will form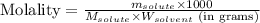
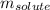 = Given mass of solute (sodium bromide) = 91.3 g
= Given mass of solute (sodium bromide) = 91.3 g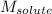 = Molar mass of solute (sodium bromide) = 103 g/mol
= Molar mass of solute (sodium bromide) = 103 g/mol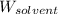 = Mass of solvent (water) = 115 g
= Mass of solvent (water) = 115 g


