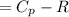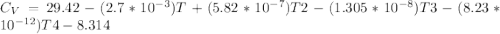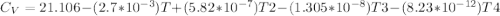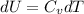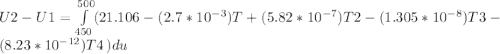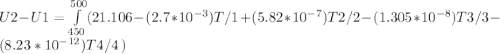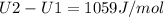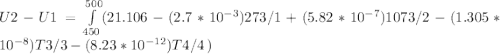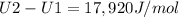The ideal gas heat capacity of nitrogen varies with temperature. It is given by:
Cp = 29.42 -...

Chemistry, 10.02.2020 23:04 steventhecool22
The ideal gas heat capacity of nitrogen varies with temperature. It is given by:
Cp = 29.42 - (2.170*10^-3 ) T + (0.0582*10^-5 ) T2 + (1.305*10^-8 ) T3 – (0.823*10^-11) T4
T in K and Cp in Joule/(mole-K). Assuming that N2 is an ideal gas:
A) How much internal energy (per mole) must be added to nitrogen to increase its temperature from 450 to 500 K. B) Repeat part A for an initial temperature of 273 K and final temperature of 1073 K.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1

You know the right answer?
Questions

Computers and Technology, 13.04.2020 23:01



Biology, 13.04.2020 23:01

Health, 13.04.2020 23:01

Mathematics, 13.04.2020 23:01





English, 13.04.2020 23:01



History, 13.04.2020 23:01




Mathematics, 13.04.2020 23:01

Health, 13.04.2020 23:01

Mathematics, 13.04.2020 23:01

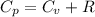
 from above if we make
from above if we make