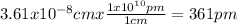Chemistry, 10.02.2020 23:31 jeffreystarks
Copper crystallizes in a face-centered cubic lattice (the Cu atoms are at the lattice points and at the face centers). If the density of the metal is 8.96 g/cm3, what is the unit cell edge length in pm? × 10 pm

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3

Chemistry, 23.06.2019 07:00
4. glenn andrews recently bought a motorcycle for $3,950. if he had to pay 6% sales tax on the bike, what was the total cost of the motorcycle?
Answers: 1
You know the right answer?
Copper crystallizes in a face-centered cubic lattice (the Cu atoms are at the lattice points and at...
Questions

Mathematics, 01.06.2020 18:59

English, 01.06.2020 18:59



Mathematics, 01.06.2020 18:59



English, 01.06.2020 18:59




Mathematics, 01.06.2020 18:59


French, 01.06.2020 18:59

Mathematics, 01.06.2020 18:59

Physics, 01.06.2020 18:59

Mathematics, 01.06.2020 18:59

Biology, 01.06.2020 18:59


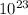 atoms/mol
atoms/mol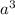 , which can be obtained through the relationship:
, which can be obtained through the relationship: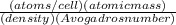
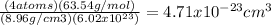
![\sqrt[3]{4.71x10^{-23}cm^{3} }=3.61x10^{-8}cm](/tpl/images/0505/4719/2ee6e.png)
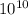 pm
pm