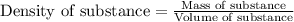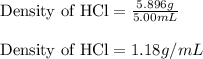Chemistry, 11.02.2020 00:09 ichabella2010
Experimental Procedure, Part C.3. The mass of a beaker is 5.333g. After 5.00mL of a concentrated hydrochloric acid solution is pipetted into the beaker, the combined mass of the beaker and the hydrochloric acid sample is 11.229g. From the data, what is the measured density of the hydrochloric acid solution?

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
You know the right answer?
Experimental Procedure, Part C.3. The mass of a beaker is 5.333g. After 5.00mL of a concentrated hyd...
Questions

Mathematics, 01.10.2019 22:00

Mathematics, 01.10.2019 22:00

English, 01.10.2019 22:00




Mathematics, 01.10.2019 22:00

Mathematics, 01.10.2019 22:00








Chemistry, 01.10.2019 22:00


Biology, 01.10.2019 22:00

Spanish, 01.10.2019 22:00

History, 01.10.2019 22:00