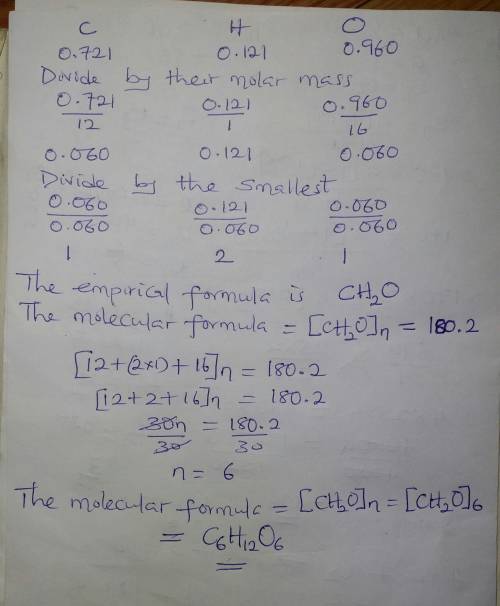Chemistry, 11.02.2020 00:34 bikerhomie
4. A 1.802 gram sample of unknown compound contains 0.721 grams of carbon, 0.121 grams of hydrogen and 0.960 grams of oxygen. The compound has a molar mass of 180.2 g/mol. Determine the empirical formula of the compound and the molecular formula.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 14:00
How can a ringing telephone can be heard through a closed door
Answers: 1

You know the right answer?
4. A 1.802 gram sample of unknown compound contains 0.721 grams of carbon, 0.121 grams of hydrogen a...
Questions

Mathematics, 10.11.2020 23:30

Mathematics, 10.11.2020 23:30


Social Studies, 10.11.2020 23:30

Mathematics, 10.11.2020 23:30


Chemistry, 10.11.2020 23:30





Chemistry, 10.11.2020 23:30

Physics, 10.11.2020 23:30



English, 10.11.2020 23:30


Mathematics, 10.11.2020 23:30

Mathematics, 10.11.2020 23:30

Mathematics, 10.11.2020 23:30