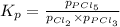Chemistry, 11.02.2020 00:56 cia196785920
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas. pcl3(g) + cl2(g) equilibrium reaction arrow pcl5(g) a gas vessel is charged with a mixture of pcl3(g) and cl2(g), which is allowed to equilibriate at 450 k. at equilibrium the partial pressures of the three gases are ppcl3 = 0.124 atm, pcl2 = 0.157 atm, and ppcl5 = 1.30 atm.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Pbco3 –> pbo+ co2. how many liters of carbon dioxide gas is produced from the decomposition of 32 grams of lead (ll) carbonate?
Answers: 1

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2


Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1
You know the right answer?
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas. pcl3(g) + cl...
Questions

Social Studies, 21.10.2021 14:00




Geography, 21.10.2021 14:00






Mathematics, 21.10.2021 14:00





Mathematics, 21.10.2021 14:00



Mathematics, 21.10.2021 14:00

English, 21.10.2021 14:00

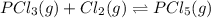
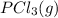 and
and 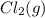 , which is allowed to equilibriate at 450 K. At equilibrium the partial pressures of the three gases are
, which is allowed to equilibriate at 450 K. At equilibrium the partial pressures of the three gases are 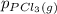 = 0.126 atm ,
= 0.126 atm , 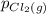 = 0.156 atm , and
= 0.156 atm , and 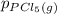 = 1.60 atm. What is the value of
= 1.60 atm. What is the value of 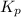 at this temperature?
at this temperature?