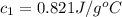Chemistry, 11.02.2020 01:14 treseanthegreat
A piece of unknown metal with mass 30 g is heated to 110.0 °C and dropped into 100.0 g of water at 20.0 °C. The final temperature of the system is 25.0 °C. Determine the specific heat of the metal.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

You know the right answer?
A piece of unknown metal with mass 30 g is heated to 110.0 °C and dropped into 100.0 g of water at 2...
Questions

Biology, 26.07.2019 08:40



History, 26.07.2019 08:40

Mathematics, 26.07.2019 08:40





Arts, 26.07.2019 08:40




Arts, 26.07.2019 08:40

History, 26.07.2019 08:40




Biology, 26.07.2019 08:40

Chemistry, 26.07.2019 08:40

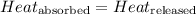
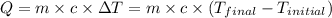
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0505/6952/09236.png) ......(1)
......(1)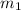 = mass of metal = 30 g
= mass of metal = 30 g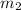 = mass of water = 100 g
= mass of water = 100 g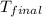 = final temperature = 25°C
= final temperature = 25°C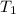 = initial temperature of metal = 110°C
= initial temperature of metal = 110°C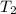 = initial temperature of water = 20.0°C
= initial temperature of water = 20.0°C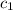 = specific heat of metal = ?
= specific heat of metal = ?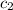 = specific heat of water = 4.186 J/g°C
= specific heat of water = 4.186 J/g°C![30\times c_1\times (25-110)=-[100\times 4.186\times (25-20)]](/tpl/images/0505/6952/ee389.png)