Dinitrogen tetraoxide is a colorless gas at room temperature. It can dissociate into nitrogen dioxide, which is a reddish brown gas.
N2O4(g) <> 2 NO2(g)
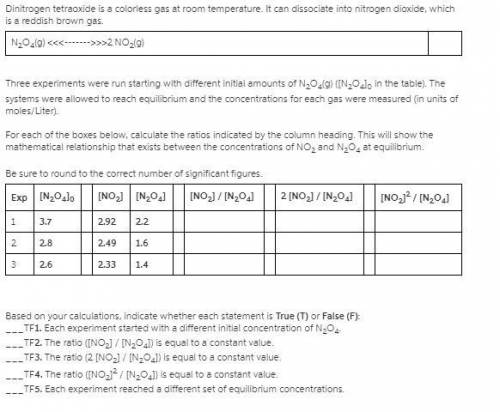
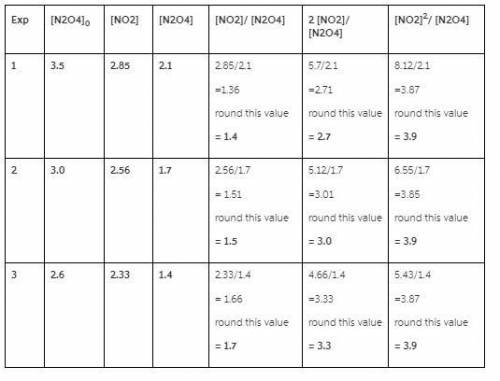
Three experiments were run starting with different initial amounts of N2O4(g) ([N2O4]0 in the table). The systems were allowed to reach equilibrium and the concentrations for each gas were measured (in units of moles/Liter).
1. For each of below, calculate the ratios indicated by the column heading. This will show the mathematical relationship that exists between the concentrations of NO2 and N2O4 at equilibrium.
Be sure to round to the correct number of significant figures.
Exp [N2O4]0 [NO2] [N2O4] [NO2] / [N2O4] 2 [NO2] / [N2O4] [NO2]2 / [N2O4]
1 3.7 2.92 2.2
2 3.0 2.56 1.7
3 2.1 2.06 1.1
2. Based on your calculations, indicate whether each statement is True (T) or False (F):
1. Each experiment started with a different initial concentration of N2O4.
2. The ratio ([NO2] / [N2O4]) is equal to a constant value.
3. The ratio (2 [NO2] / [N2O4]) is equal to a constant value.
4. The ratio ([NO2]2 / [N2O4]) is equal to a constant value.
5. Each experiment reached a different set of equilibrium concentrations.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3


Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
Dinitrogen tetraoxide is a colorless gas at room temperature. It can dissociate into nitrogen dioxid...
Questions

Mathematics, 14.12.2021 23:40

English, 14.12.2021 23:40


English, 14.12.2021 23:40

Health, 14.12.2021 23:40

Social Studies, 14.12.2021 23:40


Biology, 14.12.2021 23:40

Arts, 14.12.2021 23:40




Advanced Placement (AP), 14.12.2021 23:40

Mathematics, 14.12.2021 23:40

Physics, 14.12.2021 23:40

Mathematics, 14.12.2021 23:40

Mathematics, 14.12.2021 23:40

Mathematics, 14.12.2021 23:40

Mathematics, 14.12.2021 23:40

![[NO2]^{2}/ [N2O4]](/tpl/images/0505/8775/308c2.png) in all three experiment have same value 3.9, 3.9 & 3.9 is a constant value.
in all three experiment have same value 3.9, 3.9 & 3.9 is a constant value.