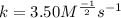Consider the equation:
CHCl3(g)+Cl2(g) > CCl4(g)+HCl(g)
The initial rate of the reac...

Chemistry, 11.02.2020 03:01 carter4026
Consider the equation:
CHCl3(g)+Cl2(g) > CCl4(g)+HCl(g)
The initial rate of the reaction is measured at several different concentrations of the reactants with the following results: [CHCl3](M) [Cl2](M) Initial rate (M/s)
0.010 0.010 0.0035
0.020 0.010 0.0069
0.020 0.020 0.0098
0.040 0.040 0.027
1. From the data, choose the correct rate law for the reaction.
Rate = k[CHCl3][Cl2]^2Rate = k[CHCl3]^2[Cl2]^1/2Rate = k[CHCl3][Cl2]Rate = k[CHCl3][Cl2]^1/2
2. the rate constant (k) for the reaction. Express your answer using three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
A3.37-mg sample of protein was chemically digested to convert its nitrogen into ammonia and then diluted to 100.0 ml. then 10.0 ml of this solution was placed in a 50-ml volumetric flask and treated with 5 ml of phenol solution plus 2 ml of sodium hypochlorite solution. the sample was diluted to 50.0 ml, and the absorbance at 625 nm was measured in a 1.00-cm cuvette and found to be 0.486. for reference, a standard solution was prepared from 10.0 mg of nh4cl (molar mass = 53.49 grams/mole) dissolved in 1.00 l of water. then 10.0 ml of this standard was placed in a 50-ml volumetric flask, treated in the same manner as the unknown, and the absorbance found to be 0.323. finally, a reagent blank was prepared using distilled water in place of unknown, it was treated in the same manner as the unknown, and the absorbance found to be 0.076. calculate the weight percent of nitrogen in the protein.
Answers: 1



Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
Questions

Mathematics, 16.07.2019 03:00





English, 16.07.2019 03:00

Mathematics, 16.07.2019 03:00

Mathematics, 16.07.2019 03:00

Mathematics, 16.07.2019 03:00


Mathematics, 16.07.2019 03:00




Computers and Technology, 16.07.2019 03:00





Mathematics, 16.07.2019 03:00

![Rate=k[CHCl_3]^1[Cl_2]^\frac{1}{2}](/tpl/images/0505/9699/bea07.png)
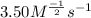
![rate=k[CHCl_3]^x[Cl_2]^y](/tpl/images/0505/9699/ba01c.png)
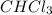
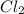
![0.0035=k[0.010]^x[0.010]^y](/tpl/images/0505/9699/b387a.png) (1)
(1)
![0.0069=k[0.020]^x[0.010]^y](/tpl/images/0505/9699/ae150.png) (2)
(2)
![\frac{0.0069}{0.035}=\frac{k[0.020]^x[0.010]^y}{k[0.010]^x[0.010]^y}](/tpl/images/0505/9699/79be9.png)
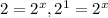 therefore x=1.
therefore x=1.![0.0098=k[0.020]^x[0.020]^y](/tpl/images/0505/9699/46f00.png) (4)
(4)
![\frac{0.0098}{0.0069}=\frac{k[0.020]^x[0.020]^y}{k[0.020]^x[0.010]^y}](/tpl/images/0505/9699/470b5.png)
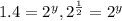 therefore
therefore 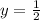
![rate=k[CHCl_3]^1[Cl_2]^\frac{1}{2}](/tpl/images/0505/9699/cc117.png)
![0.0035=k[0.010]^1[0.010]^\frac{1}{2}](/tpl/images/0505/9699/a917e.png)