
Chemistry, 11.02.2020 03:23 kookycookiefanx
Suppose a certain biologically important reaction is quite slow at physiological temperature (37 °C) in the absence of a catalyst. Assuming that the frequency factor remains the same, by how much must an enzyme lower the activation energy of the reaction to achieve a million- fold (1.0 x 106) increase in the reaction rate constant?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 23.06.2019 14:30
If 125 cal of heat is applied to a 60.0-g piece of copper at 25.0 ? c , what will the final temperature be? the specific heat of copper is 0.0920 cal/(g? ? c) .
Answers: 1

Chemistry, 23.06.2019 15:00
The atoms in a have a definite volume, but move quickly enough to overcome the forces of attraction between them. a. solid b.liquid c.gas
Answers: 2

Chemistry, 23.06.2019 15:30
The amount of iron in ore can be quantitatively determined by titrating a solution of the unknown with a standard solution of dichromate, cr2o72−. the net ionic equation is 6fe2+(aq)+cr2o72−(aq)+14h+(aq)→6fe3+(aq)+2cr3+(aq)+7h2o(aq) part a the titration of 25.0 ml of an iron(ii) solution required 18.0 ml of a 0.230 m solution of dichromate to reach the equivalence point. what is the molarity of the iron(ii) solution?
Answers: 1
You know the right answer?
Suppose a certain biologically important reaction is quite slow at physiological temperature (37 °C)...
Questions




Chemistry, 28.06.2019 11:00


Mathematics, 28.06.2019 11:00

Mathematics, 28.06.2019 11:00

Biology, 28.06.2019 11:00



Mathematics, 28.06.2019 11:00

Mathematics, 28.06.2019 11:00

Biology, 28.06.2019 11:00

History, 28.06.2019 11:00


Geography, 28.06.2019 11:00




History, 28.06.2019 11:00

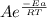
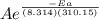
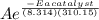
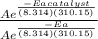 = 1.0 x 10⁶ by taking natural logarithm of both sides, we have
= 1.0 x 10⁶ by taking natural logarithm of both sides, we have


