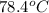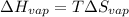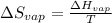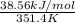Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1


Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
The boiling point of ethanol is 78.4 °C, and the enthalpy change for the conversion of liquid to vap...
Questions





Physics, 26.04.2022 22:00








Mathematics, 26.04.2022 23:00



Mathematics, 26.04.2022 23:10


Computers and Technology, 26.04.2022 23:10

English, 26.04.2022 23:10

Mathematics, 26.04.2022 23:20

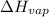 = 38.56 kJ/mol
= 38.56 kJ/mol