Chemistry, 11.02.2020 05:24 alexmodersks3055
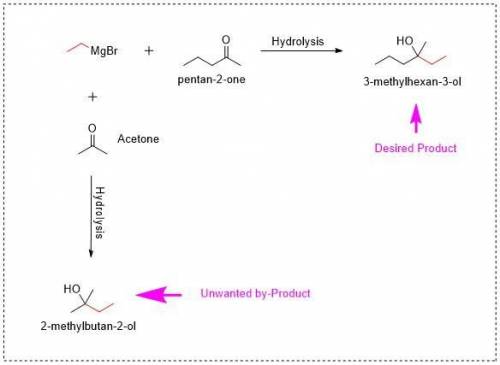
A Grignard reagent and a ketone are reacted in ether solution and, followed by an acid workup, form a tertiary alcohol. Recall that Grignard reactions must be scrupulously dry in order to work effectively. A common method of drying glassware is to rinse with acetone prior to use.
1. Why is rinsing with acetone not suitable for the reaction stated in the question.
a) Magnesium does not dissolve in acetone.
b) Water dissolves in acetone. Adding acetone will add water to the reaction flask.
c) Magnesium dissolves in acetone. Adding acetone will remove a vital reactant from the flask.
d) Acetone is a ketone. Grignard reagents will react with acetone to make an unwanted byproduct.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Fugu, also known as puffer fish, is a sushi delicacy that can also be lethal. puffer fish contain a powerful toxin that can kill an adult a few hours after ingestion. sushi chefs who prepare fugu must be specially trained because any contamination of the toxin-free areas of the fish can be deadly. recently this toxin has been put to good use, as scientists have discovered that a purified form of it can treat severe pain in cancer patients. this recent scientific discovery would fall under which area of chemistry? applied biochemistry pure organic chemistry pure physical chemistry applied inorganic chemistry
Answers: 1

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1

Chemistry, 23.06.2019 08:00
The biosphere of the earth is made up of . a. inorganic b. organic
Answers: 2
You know the right answer?
A Grignard reagent and a ketone are reacted in ether solution and, followed by an acid workup, form...
Questions















Biology, 26.11.2019 17:31

Social Studies, 26.11.2019 17:31

Health, 26.11.2019 17:31

History, 26.11.2019 17:31


English, 26.11.2019 17:31