
Chemistry, 11.02.2020 06:00 mariahdelossantos031
Calculate the energy required to produce 7.00 mol Cl2O7 on the basis of the following balanced equation. 2Cl2(g) + 7O2(g) + 130 kcal --> 2Cl2O7(g) Select one: a. 7.00 kcal b. 65 kcal c. 130 kcal d. 455 kcal

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
Calculate the energy required to produce 7.00 mol Cl2O7 on the basis of the following balanced equat...
Questions

Mathematics, 12.05.2021 22:30

Mathematics, 12.05.2021 22:30


Mathematics, 12.05.2021 22:30

Mathematics, 12.05.2021 22:30


Mathematics, 12.05.2021 22:30



English, 12.05.2021 22:30

History, 12.05.2021 22:30


Physics, 12.05.2021 22:30



English, 12.05.2021 22:30




Mathematics, 12.05.2021 22:30

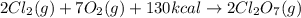
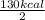
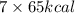
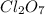 on the basis of given reaction is 455 kcal.
on the basis of given reaction is 455 kcal.

