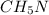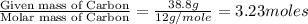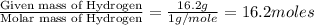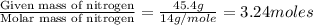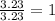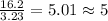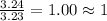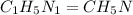Chemistry, 11.02.2020 17:21 olivernolasco23
A laboratory analysis of a sample finds it is composed of 38.8% carbon, 16.2% hydrogen, and 45.1% nitrogen. What is its empirical formula? Give your answer in the form C#H#N#, where the number following the element’s symbol corresponds to the subscript in the formula.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2


Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
A laboratory analysis of a sample finds it is composed of 38.8% carbon, 16.2% hydrogen, and 45.1% ni...
Questions

Social Studies, 15.04.2021 01:20


Mathematics, 15.04.2021 01:20

English, 15.04.2021 01:20

Mathematics, 15.04.2021 01:20

Mathematics, 15.04.2021 01:20


Mathematics, 15.04.2021 01:20

Mathematics, 15.04.2021 01:20

Mathematics, 15.04.2021 01:20

Mathematics, 15.04.2021 01:20

Mathematics, 15.04.2021 01:20

History, 15.04.2021 01:20



Mathematics, 15.04.2021 01:20

English, 15.04.2021 01:20


Mathematics, 15.04.2021 01:20