
Chemistry, 11.02.2020 18:45 Theresab2021
The decomposition of N2O5 in solution in carbon tetrachloride proceeds via the reaction 2 N2O5(soln) → 4 NO2(soln) + O2(soln) The reaction is first order and has a rate constant of 4.82 × 10-3 s-1 at 64°C. If the reaction is initiated with 0.085 mol in a 1.00-L vessel, how many moles remain after 151 s?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3

Chemistry, 23.06.2019 07:30
Which statement explains which thermometer is more appropriate to measure the temperature of a liquid at 43.6 degrees celsius a) thermometer a, because it measures temperature more accurately than thermometer b b) thermometer b, because it measures temperature more accurately than thermometer a c) thermometer a, because it measures temperature more precisely than thermometer b d) thermometer b, because it measures temperature more precisely than thermometer a
Answers: 2
You know the right answer?
The decomposition of N2O5 in solution in carbon tetrachloride proceeds via the reaction 2 N2O5(soln)...
Questions

Mathematics, 12.08.2020 09:01

Biology, 12.08.2020 09:01


English, 12.08.2020 09:01

Mathematics, 12.08.2020 09:01


English, 12.08.2020 09:01


Mathematics, 12.08.2020 09:01

English, 12.08.2020 09:01

Mathematics, 12.08.2020 09:01

Mathematics, 12.08.2020 09:01

Mathematics, 12.08.2020 09:01

Biology, 12.08.2020 09:01

Mathematics, 12.08.2020 09:01

Health, 12.08.2020 09:01

History, 12.08.2020 09:01


Mathematics, 12.08.2020 09:01

Biology, 12.08.2020 09:01

![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0506/7634/f1041.png)
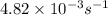
![[A_o]](/tpl/images/0506/7634/dc622.png) = initial amount of the reactant = 0.085 moles
= initial amount of the reactant = 0.085 moles![4.82\times 10^{-3}=\frac{2.303}{151}\log\frac{0.085}{[A]}](/tpl/images/0506/7634/8f69f.png)
![[A]=0.041moles](/tpl/images/0506/7634/e3fb9.png)


