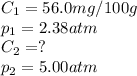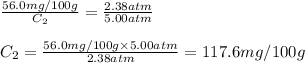Chemistry, 11.02.2020 19:23 Whitehouse9
At a certain temperature, the solubility of N₂ gas in water at 2.38 atm is 56.0 mg of N₂ gas/100 g water. Calculate the solubility of N₂ gas in water, at the same temperature, if the partial pressure of N₂ gas over the solution is increased from 2.38 atm to 5.00 atm.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Noble gases are the most reactive elements on the periodic table. a. true b. false
Answers: 2

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
You know the right answer?
At a certain temperature, the solubility of N₂ gas in water at 2.38 atm is 56.0 mg of N₂ gas/100 g w...
Questions

Mathematics, 19.12.2020 05:30

Biology, 19.12.2020 05:30

Mathematics, 19.12.2020 05:30



Mathematics, 19.12.2020 05:30



Mathematics, 19.12.2020 05:30


Mathematics, 19.12.2020 05:30

Mathematics, 19.12.2020 05:30


Mathematics, 19.12.2020 05:30

Geography, 19.12.2020 05:30

Mathematics, 19.12.2020 05:30

Mathematics, 19.12.2020 05:30

Mathematics, 19.12.2020 05:30

Chemistry, 19.12.2020 05:30

Mathematics, 19.12.2020 05:30

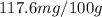
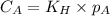
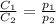
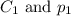 are the initial concentration and partial pressure of nitrogen gas
are the initial concentration and partial pressure of nitrogen gas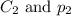 are the final concentration and partial pressure of nitrogen gas
are the final concentration and partial pressure of nitrogen gas