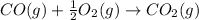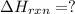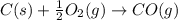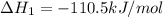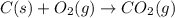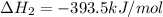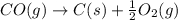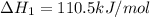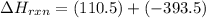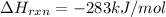CO(g) + 12 O2(g) → CO2(g)The combustion of carbon monoxide is represented by the equation above.(a) Determine the value of the standard enthalpy change, ∆HrxnD , for the combustion of CO(g) at 298 Kusing the following information. C(s) + 12 O2(g) → CO(g) ∆H298D = − 110.5 kJ mol−1 C(s) + O2(g) → CO2(g) ∆H298D = − 393.5 kJ mol−1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1


Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1
You know the right answer?
CO(g) + 12 O2(g) → CO2(g)The combustion of carbon monoxide is represented by the equation above.(a)...
Questions

English, 07.10.2020 14:01





Social Studies, 07.10.2020 14:01


Arts, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01

Medicine, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01


Mathematics, 07.10.2020 14:01


Arts, 07.10.2020 14:01

Geography, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01


Mathematics, 07.10.2020 14:01

Mathematics, 07.10.2020 14:01

 will be,
will be,