
Chemistry, 11.02.2020 20:55 brummy309506
Nitrogen and hydrogen react to form ammonia according to the following balanced equation: N2(g) + 3H2(g) → 2NH3(g) Calculate the number of moles of hydrogen required to react with 0.0763 mole of nitrogen, and the number of moles of ammonia that will form.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2
You know the right answer?
Nitrogen and hydrogen react to form ammonia according to the following balanced equation: N2(g) + 3H...
Questions










Biology, 25.11.2019 18:31


English, 25.11.2019 18:31

Mathematics, 25.11.2019 18:31

Geography, 25.11.2019 18:31




History, 25.11.2019 18:31

Mathematics, 25.11.2019 18:31

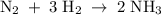
 3 moles of Hydrogen
3 moles of Hydrogen

