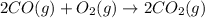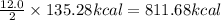Chemistry, 11.02.2020 20:55 7letters22
Carbon monoxide reacts with oxygen to form carbon dioxide by the following reaction: 2 CO(g) + O2(g) → 2 CO2(g)∆H for this reaction is −135.28 kcal. How much heat would be released if 12.0 moles of carbon monoxide reacted with sufficient oxygen to produce carbon dioxide? Use only the information provided in this question. 1. 270.56 kcal 2. 1623.36 kcal 3. 811.68 kcal 4. 405.84 kcal 5. 541.12 kcal 6. 135.28 kcal

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Measuring which physical property is most likely to produce the most precise results when trying to identify a substance
Answers: 1

Chemistry, 21.06.2019 17:00
Write the complete balanced equation for the reaction between lead (iv) oxide (pbo2) and water (h2o).
Answers: 1

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1
You know the right answer?
Carbon monoxide reacts with oxygen to form carbon dioxide by the following reaction: 2 CO(g) + O2(g)...
Questions

Mathematics, 08.01.2021 04:10



Mathematics, 08.01.2021 04:10





Mathematics, 08.01.2021 04:10





English, 08.01.2021 04:10

History, 08.01.2021 04:10

Mathematics, 08.01.2021 04:10

Social Studies, 08.01.2021 04:10

Mathematics, 08.01.2021 04:10

Mathematics, 08.01.2021 04:10

Mathematics, 08.01.2021 04:20