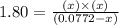Chemistry, 11.02.2020 21:05 lilpeepxliltracy
Phosphorus pentachloride decomposes according to the chemical equation PCl 5 ( g ) − ⇀ ↽ − PCl 3 ( g ) + Cl 2 ( g ) K c = 1.80 at 250 ∘ C A 0.166 mol sample of PCl 5 ( g ) is injected into an empty 2.15 L reaction vessel held at 250 ∘ C. Calculate the concentrations of PCl 5 ( g ) and PCl 3 ( g ) at equilibrium.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3
You know the right answer?
Phosphorus pentachloride decomposes according to the chemical equation PCl 5 ( g ) − ⇀ ↽ − PCl 3 ( g...
Questions

Mathematics, 30.09.2021 01:00


Mathematics, 30.09.2021 01:00


Mathematics, 30.09.2021 01:00


English, 30.09.2021 01:00

Mathematics, 30.09.2021 01:00

Mathematics, 30.09.2021 01:00


Chemistry, 30.09.2021 01:00







Mathematics, 30.09.2021 01:00

SAT, 30.09.2021 01:00

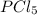 and
and 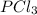 at equilibrium is, 0.0031 M and 0.0741 M respectively.
at equilibrium is, 0.0031 M and 0.0741 M respectively.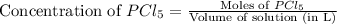
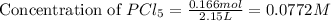
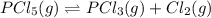
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0507/1118/73fe0.png)