Chemistry, 11.02.2020 21:27 litzyguzman13
In a zero order reaction, it takes 342 seconds for 75% of a hypothetical reaction to decompose. Determine the half-life t1/2} in units of seconds. Do not enter units with your numerical answer.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
In a zero order reaction, it takes 342 seconds for 75% of a hypothetical reaction to decompose. Dete...
Questions

Mathematics, 16.09.2019 14:50

Biology, 16.09.2019 14:50



Biology, 16.09.2019 14:50

History, 16.09.2019 14:50

Chemistry, 16.09.2019 14:50

Biology, 16.09.2019 14:50


Mathematics, 16.09.2019 14:50



Biology, 16.09.2019 14:50

Computers and Technology, 16.09.2019 14:50

Biology, 16.09.2019 14:50

Geography, 16.09.2019 14:50

History, 16.09.2019 14:50




![\ln [A]=-kt+\ln [A_o]](/tpl/images/0507/1434/bdc3f.png)
![[A_o]](/tpl/images/0507/1434/dc622.png) = let initial concentration = 100
= let initial concentration = 100![[A]](/tpl/images/0507/1434/6aa06.png) = final concentration = 25
= final concentration = 25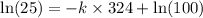
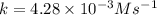
![t_{1/2}=\frac{[A_o]}{2k}](/tpl/images/0507/1434/b5b11.png)