Chemistry, 11.02.2020 22:25 xthom001ow7go3
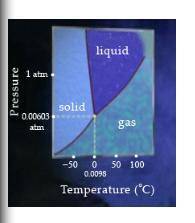
The triple point of water is 0.0098%u2218C at 0.00603 atm (4.58 torr). At the triple point, ice, water, and water vapor exist in equilibrium with each other.
Complete the following sentences to identify the process that ice, water, or water vapor may undergo if either the temperature or the pressure is increased.
Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer.
FREEZE 1. If ice is heated at a constant pressure of 0.00512 atm, it will.
CONDENSE 2. If ice is heated at a constant pressure of 1 atm, it will.
MELT 3. If the pressure of water vapor is increased at a constant pressure of 100 degrees Celsius, it will.
SUBLIME 4. If the pressure of water vapor is increased at a constant pressure of -50 degrees Celsius, it will__.
VAPORIZE
DEPOSIT

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1

Chemistry, 23.06.2019 10:00
Lord kelvin described the concept of absolute zero temperature and the laws relating to the change of thermal energy during chemical reactions what type of chemist would he be considered today
Answers: 1
You know the right answer?
The triple point of water is 0.0098%u2218C at 0.00603 atm (4.58 torr). At the triple point, ice, wat...
Questions


Mathematics, 18.07.2019 03:50

Mathematics, 18.07.2019 03:50



English, 18.07.2019 03:50

Mathematics, 18.07.2019 03:50



Social Studies, 18.07.2019 03:50


Mathematics, 18.07.2019 03:50

Arts, 18.07.2019 03:50





Biology, 18.07.2019 03:50