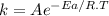The rate constant for a reaction is measured as a function of time. A plot is created by graphing ln(k) on the y-axis and 1/T on the x-axis, and a best fit line with a slope of -10,473 K is obtained. Based on this data what is the activation energy of this reaction (in kJ/mol)?

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 06:30
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
You know the right answer?
The rate constant for a reaction is measured as a function of time. A plot is created by graphing ln...
Questions

Chemistry, 11.04.2021 20:10




English, 11.04.2021 20:10

Mathematics, 11.04.2021 20:10

History, 11.04.2021 20:10



Mathematics, 11.04.2021 20:10



Mathematics, 11.04.2021 20:10

English, 11.04.2021 20:10

Mathematics, 11.04.2021 20:10

Mathematics, 11.04.2021 20:10



Geography, 11.04.2021 20:10

Computers and Technology, 11.04.2021 20:10