Chemistry, 12.02.2020 00:27 coollid876
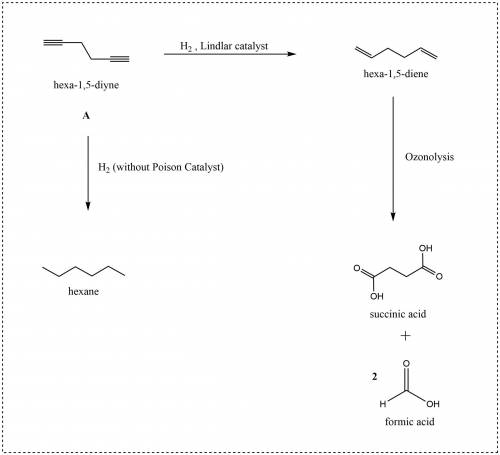
A compound A (C6H6) undergoes catalytic hydrogenation over Lindlar catalyst to give a compound B, which in turn undergoes ozonolysis followed by workup with aqueous H2O2 to yield succinic acid and two equivalents of formic acid. In the absence of a catalyst poison, hydrogenation of A gives hexane. Propose a structure for compound A.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
A compound A (C6H6) undergoes catalytic hydrogenation over Lindlar catalyst to give a compound B, wh...
Questions




Biology, 24.04.2020 18:11


History, 24.04.2020 18:11




Mathematics, 24.04.2020 18:11




Mathematics, 24.04.2020 18:12


Biology, 24.04.2020 18:12

History, 24.04.2020 18:12

Mathematics, 24.04.2020 18:12