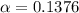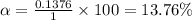Chemistry, 12.02.2020 00:30 bbyjean9974
A student prepares a 0.61mM aqueous solution of propionic acid C2H5CO2H. Calculate the fraction of propionic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 06:00
What does it mean for something to be dissolved in watera- it is submerged in water moleculesb-it is stirred in the water moleculesc- it is surrounded by water molecules d-it has water molecules added to it
Answers: 2
You know the right answer?
A student prepares a 0.61mM aqueous solution of propionic acid C2H5CO2H. Calculate the fraction of p...
Questions

Computers and Technology, 28.08.2020 21:01


English, 28.08.2020 21:01

History, 28.08.2020 21:01



History, 28.08.2020 21:01

Chemistry, 28.08.2020 21:01

Physics, 28.08.2020 21:01

Mathematics, 28.08.2020 21:01

Chemistry, 28.08.2020 21:01


Health, 28.08.2020 21:01

Mathematics, 28.08.2020 21:01


Mathematics, 28.08.2020 21:01

Mathematics, 28.08.2020 21:01

Mathematics, 28.08.2020 21:01


History, 28.08.2020 21:01

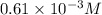
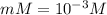
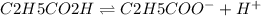
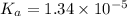
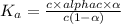
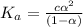
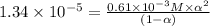
 :
: