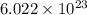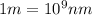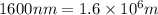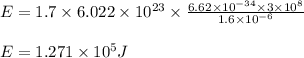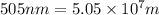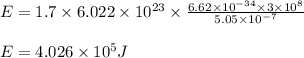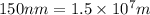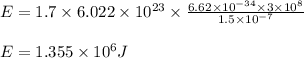Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:04
What is the smallest component or the most basic building block of any element ? a. an atom, b.a compound c.gas d.element
Answers: 1

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
You know the right answer?
Determine the energy of 1.70 mol of photons for each of the following kinds of light. (Assume three...
Questions




English, 02.06.2021 02:00


Mathematics, 02.06.2021 02:00


English, 02.06.2021 02:00



History, 02.06.2021 02:00

Mathematics, 02.06.2021 02:00


Mathematics, 02.06.2021 02:00


Mathematics, 02.06.2021 02:00

Mathematics, 02.06.2021 02:00


English, 02.06.2021 02:00

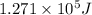

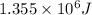
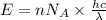 ......(1)
......(1)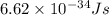
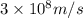
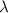 = wavelength of light
= wavelength of light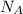 = Avogadro's number =
= Avogadro's number =