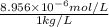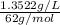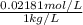Chemistry, 12.02.2020 02:17 ethangorrell67
High concentrations of ammonia (NH3), nitrite ion, and nitrate ion in water can kill fish. Lethal concentrations of these species for rainbow trout are approximately 1.002 mg/L, 0.412 mg/L, and 1352.2 mg/L, respectively. Express these concentrations in molality units, assuming a solution density of 1.00 g/mL. a. m ammoniab. m nitrite ironc. m nitrate ion

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1


You know the right answer?
High concentrations of ammonia (NH3), nitrite ion, and nitrate ion in water can kill fish. Lethal co...
Questions

English, 14.04.2021 16:00





Social Studies, 14.04.2021 16:00


Computers and Technology, 14.04.2021 16:00

Mathematics, 14.04.2021 16:00

Mathematics, 14.04.2021 16:00






Health, 14.04.2021 16:00

Mathematics, 14.04.2021 16:00

Mathematics, 14.04.2021 16:00

English, 14.04.2021 16:00

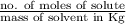
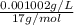
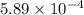 mol/L
mol/L
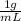 = 1 kg/L
= 1 kg/L
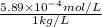
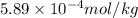
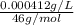
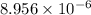 mol/L
mol/L