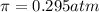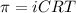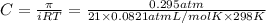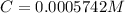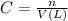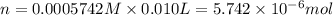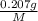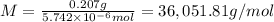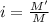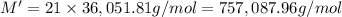Chemistry, 12.02.2020 02:53 dtilton2003
A protein has been isolated as a salt with the formula Na20P (this notation means that there are 20 Na+ ions associated with a negatively charged protein P20−). The osmotic pressure of a 10.0 mL solution containing 0.207 g of the protein is 0.295 atm at 25.0°C. (a) Calculate the molar mass of the protein from these data. g/mol (b) Calculate the actual molar mass of the protein.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Which of these will change if the air in aclosed bottle is heated? abcdthe mass of the airthe composition of the airthe air pressure in the bottlethe number of air molecules in the bottle
Answers: 3

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3
You know the right answer?
A protein has been isolated as a salt with the formula Na20P (this notation means that there are 20...
Questions


Mathematics, 29.09.2019 04:30



Mathematics, 29.09.2019 04:30

English, 29.09.2019 04:30

Mathematics, 29.09.2019 04:30






History, 29.09.2019 04:30

Chemistry, 29.09.2019 04:30





History, 29.09.2019 04:30