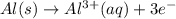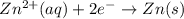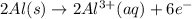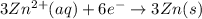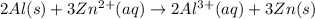The half-reactions for the oxidation-reduction reaction between Al(s) and Zn₂ (aq) are represented above. Based on the half-reactions, what is the coefficient for Al(s) if the equation for the oxidation-reduction reaction is balanced with the smallest whole-number coefficients?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
The half-reactions for the oxidation-reduction reaction between Al(s) and Zn₂ (aq) are represented a...
Questions

Mathematics, 30.10.2020 14:00


Arts, 30.10.2020 14:00


Mathematics, 30.10.2020 14:00

English, 30.10.2020 14:00

Mathematics, 30.10.2020 14:00

History, 30.10.2020 14:00

Physics, 30.10.2020 14:00

Chemistry, 30.10.2020 14:00

English, 30.10.2020 14:00

History, 30.10.2020 14:00

History, 30.10.2020 14:00





Mathematics, 30.10.2020 14:00

Computers and Technology, 30.10.2020 14:00

Biology, 30.10.2020 14:00