
Chemistry, 12.02.2020 04:39 winterblanco
1. Use VSEPR theory to decide which one of the following ions and molecules is likely to be planar. (The central atom is always first in the formula.) A. BrF3 B. H3O C. PCl3 D. SO42- E. SF4

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1
You know the right answer?
1. Use VSEPR theory to decide which one of the following ions and molecules is likely to be planar....
Questions

Mathematics, 16.11.2020 19:50

Physics, 16.11.2020 19:50


History, 16.11.2020 19:50

Mathematics, 16.11.2020 19:50


Mathematics, 16.11.2020 19:50

Advanced Placement (AP), 16.11.2020 19:50

Mathematics, 16.11.2020 19:50






Social Studies, 16.11.2020 19:50

Mathematics, 16.11.2020 19:50




Social Studies, 16.11.2020 19:50

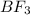
![\text{Number of electron pair}=\frac{1}{2}[V+N-C+A]](/tpl/images/0508/1323/9e987.png)
![\text{Number of electrons}=\frac{1}{2}\times [3+3]=3](/tpl/images/0508/1323/ce6da.png)
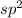 and the electronic geometry of the molecule will be trigonal planar.
and the electronic geometry of the molecule will be trigonal planar.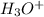
![\text{Number of electrons}=\frac{1}{2}\times [6+3-1]=4](/tpl/images/0508/1323/9514c.png)
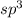 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.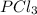
![\text{Number of electrons}=\frac{1}{2}\times [5+3]=4](/tpl/images/0508/1323/5f4d9.png)
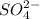
![\text{Number of electrons}=\frac{1}{2}\times [6+2]=4](/tpl/images/0508/1323/2c46b.png)
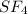
![\text{Number of electrons}=\frac{1}{2}\times [6+4]=5](/tpl/images/0508/1323/f1dca.png)
 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.

